Get HTA
Meeting the challenge of HTA…and beyond
The previous discussion consider the likely place of HTA in the reimbursement environment for tests and devices in the EU over the medium-term. Long-term, the requirements for evidence of cost-effectiveness are likely to apply to more products, more of the time, with growing expectations as to quantity and quality of evidence, and greater ability to block or limit reimbursement.
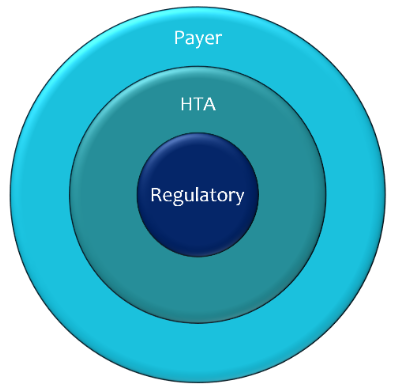
The issues you need to consider as you develop a product must include those required to satisfy the demands and get HTA. Others are determined by CE-marking regulations. There is a third group, the requirements of payers. In the uncommon cases where the positive conclusions of an HTA are binding on payers (for example, NICE’s Technology Appraisal Guidance recommendations, which must normally be funded by NHS payers within three months of publication), these are the same. In most cases, an HTA is advisory and payers are free to adopt all, some, or none of the conclusions.
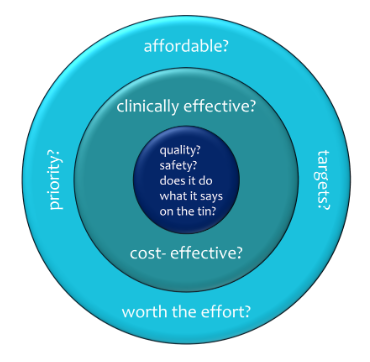
Where are payers’ interests wider than those of HTA? In such practical matters as the management effort required to implement change (moving facilities, staff, contracts, and budgets around), how likely it is that staff will change the way they work, local and national priorities (Figure 1). Most of all, health economics considers worth, not affordability. The evidence for cost-effectiveness may be solid, but there may not be the budget for it. The budget-holder may not have the resources to make an initial investment in order to making downstream savings, however good the evidence and ROI. If HTA is the fourth hurdle, practical payer concerns are a fifth hurdle.
Key to Figure 1
Nor do HTAs typically take account of the quirks of reimbursement architecture. Not everything that makes sense to a health economist does so when exposed to the imprecision, history, and arbitrary decisions which often affect money paid to providers.
The interaction of a product’s features and benefits with each country’s reimbursement system is unique, at least to each subclass of device or test. Although it is impossible to summarise in the abstract what the reimbursement climate for a range of products is going to be, it is possible to list the main parameters that are likely to be significant.
An assessment of the reimbursement environment for a product is four-pronged:
- what is the business proposition
- how does the proposition ’fit’ with the architecture of the reimbursement system
- what evidence will you need to support the reimbursement case
- what specific procedures and processes do you either have to go through or can opt to do so